Buffered chlorogallate(iii) ionic liquids and electrodeposition of gallium films
Abstract
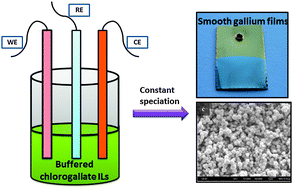
Buffering of Lewis acidic chlorometallate ionic liquids is a useful tool to modify their properties for electrochemical and catalytic applications. Lewis acidic chlorogallate(III) ionic liquids containing the 1-octyl-3-methylimidazolium cation, buffered with sodium chloride, were studied using 71Ga NMR spectroscopy and cyclic voltammetry. All the studied Lewis acidic compositions (0.50 < χGaCl3 ≤ 0.75) could be buffered to mild or moderate acidity, but not to neutrality. Electrodeposition of gallium from such buffered systems was possible, yielding deposits of improved morphology over the unbuffered ionic liquids, due to the constant melt composition maintained by the buffer. These findings were in a stark contrast with older studies on chloroaluminate(III) ionic liquids buffered with sodium chloride.

 Please wait while we load your content...
Please wait while we load your content...