CCSD(T) level interaction energy for halogen bond between pyridine and substituted iodobenzenes: origin and additivity of substituent effects†
Abstract
The CCSD(T) level interaction energies at the basis set limit (Eint) were calculated for 33
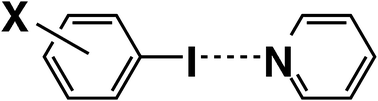
- This article is part of the themed collection: 1st International Conference on Noncovalent Interactions

 Please wait while we load your content...
Please wait while we load your content...