The fundamental chemical equation of aromaticity†
Abstract
In the search for the chemical measure of molecular aromaticity, namely the energetic gain of a cyclic delocalized structure for just being cyclic, the numerous definitions of generic aromatic stabilisation energies proposed hitherto stand as approximates of the conceptual limit devised within the framework of spectral graph theory, i.e. the topological resonance energy (TRE). After a 36 year challenge, the TRE acyclic reference of any π-cyclic molecule, originally merely defined by an abstract matching polynomial, is now given a real chemical structure: the Möbius-twisted head-to-tail metathesis cyclo-dimer of the parent ring. The original treatment at the Hückel molecular orbital level of theory can now be extended to DFT or ab initio levels. The corresponding ring opening–closing–twisting chemical transformation provides the observable basis for the measure of aromaticity under either vertical or adiabatic conditions.
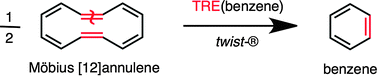

 Please wait while we load your content...
Please wait while we load your content...