Chirality recognition of the protonated serine dimer and octamer by infrared multiphoton dissociation spectroscopy†
Abstract
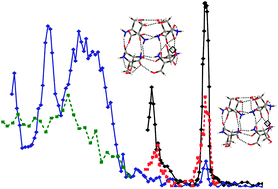
Infrared multiphoton dissociation (IRMPD) spectroscopy has been used to record IR signatures of chirality recognition in the protonated serine dimer and octamer in the 3200–3800 cm−1 region. This is the first IRMPD study to investigate the heterochiral biomolecular system by utilizing the isotope-labelled species. Noticeable differences in the homo- versus heterochiral IRMPD spectra have been obtained experimentally for both the dimer and octamer. Different dissociation patterns have been noted not only between the homo- and heterochiral octamers, but also between the two –OH stretching vibrational bands of the same chirality species. Systematic theoretical searches have been carried out to identify the most stable conformers of both the homo- and heterochiral protonated serine dimer and octamer. The final geometry optimization and harmonic vibrational calculations have been performed at the MP2/6-311++G(d,p) level for the homo- and heterochiral protonated serine dimer and at the B3LYP/6-31G(d) level for the homo- and heterochiral protonated serine octamer. For the homo- and heterochiral dimer, good agreement between the experimental and theoretical spectra has been achieved and the major conformers have been identified. For the homo- and heterochiral octamer, the main IR features observed have been satisfactorily reproduced theoretically and the dominant conformers identified. More than one main conformer has been identified for the homochiral octamer. This conclusion has been further supported by the analysis of the wavelength specific dissociation products.

 Please wait while we load your content...
Please wait while we load your content...