Steady-state macroscale voltammetry in a supercritical carbon dioxide medium†
Abstract
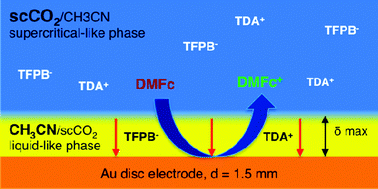
The electrochemical oxidation and reduction of decamethylferrocene is demonstrated in supercritical carbon dioxide at a macro gold disc electrode at 100 bar and 313 K. Fast mass transport effects were exhibited and the corresponding steady-state voltammetry was observed at high scan rates. A highly lipophilic room temperature ionic liquid that readily dissolved in supercritical CO2 with acetonitrile as a co-solvent was used as an electrolyte, allowing for a conducting supercritical single phase. Experimental observations along with simulation results confirmed the hypothesis that a thin layer of liquid-like phase of co-solvent is formed at the electrode surface and is restricted by a more supercritical phase of high natural convection and bulk concentration.

 Please wait while we load your content...
Please wait while we load your content...