On the limited performances of sulfone electrolytes towards the LiNi0.4Mn1.6O4 spinel
Abstract
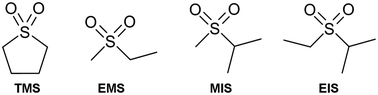
Cycling after storage of LiNi0.4Mn1.6O4/Li4Ti5O12 cells evidences lower total capacity losses for EMS-, TMS- and MIS-based electrolytes as compared to EC-based at 20 °C. The shuttle-type mechanism induced by the electrolyte oxidation is mainly present in the accumulators at this temperature, as compared to those due to the Mn2+ and Ni2+ dissolution. At 30 and 40 °C, EC is responsible for the polymer film formation on the LiNi0.4Mn1.6O4 surface, which limits the transition metal ion dissolution. This results in lower reversible capacity losses compared to sulfones, but are still important: 45% at 30 °C and 70–75% at 40 °C. XPS spectra reveal that EMS does not contribute to the surface film formation on the LiNi0.4Mn1.6O4 spinel, regardless of the cycling conditions and temperature. Only the EMC decomposition at high potential in sulfone/EMC electrolytes is responsible for an organic layer formation, which is composed of low passivating oligomers that comprise the C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functional groups. Sulfones are promising compounds to be used in high voltage Li-ion batteries thanks to their non-reactivity towards the LiNi0.4Mn1.6O4 cathode. However, this does not allow the deposition of surface films that would have enabled stopping the Mn2+ and Ni2+ dissolution in the electrolyte. This is responsible for degraded performances of LiNi0.4Mn1.6O4/Li4Ti5O12 cells as compared to EC-based electrolytes over ambient temperatures, especially at 30 °C.
O functional groups. Sulfones are promising compounds to be used in high voltage Li-ion batteries thanks to their non-reactivity towards the LiNi0.4Mn1.6O4 cathode. However, this does not allow the deposition of surface films that would have enabled stopping the Mn2+ and Ni2+ dissolution in the electrolyte. This is responsible for degraded performances of LiNi0.4Mn1.6O4/Li4Ti5O12 cells as compared to EC-based electrolytes over ambient temperatures, especially at 30 °C.

 Please wait while we load your content...
Please wait while we load your content...