Gas-phase spectroscopy of protonated adenine, adenosine 5′-monophosphate and monohydrated ions
Abstract
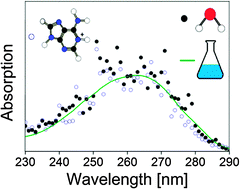
Microsolvation of chromophore ions commonly has large effects on their electronic structure and as a result on their optical absorption spectra. Here spectroscopy of protonated adenine (AdeH+) and its complex with one water molecule isolated in vacuo was done using a home-built mass spectrometer in combination with a tuneable pulsed laser system. Experiments also included the protonated adenosine 5′-monophosphate nucleotide (AMPH+). In the case of bare AdeH+ ions, one-photon absorption leads to four dominant fragment ions corresponding to ammonium and ions formed after loss of either NH3, HCN, or NH2CN. The yields of these were measured as a function of the wavelength of the light from 210 nm to 300 nm, and they were combined to obtain the total photoinduced dissociation at each wavelength (i.e., action spectrum). A broad band between 230 nm and 290 nm and the tail of a band with maximum below 210 nm (high-energy band) are seen. In the case of AdeH+(H2O), the dominant dissociation channel after photoexcitation in the low-energy band was simply loss of H2O while photodissociation of protonated AMP revealed two dominant dissociation channels associated with the formation of either AdeH+ or loss of H3PO4. The action spectra of AdeH+, AdeH+(H2O), and AMPH+ are almost identical in the 230–290 nm region, and they resemble the absorption spectrum of protonated adenine in aqueous solution recorded at low pH. Hence from our work it is firmly established that the lowest-energy transitions are independent of the surroundings.

 Please wait while we load your content...
Please wait while we load your content...