Water adsorption and dissociation on Ni surface: Effects of steps, dopants, coverage and self-aggregation†
Abstract
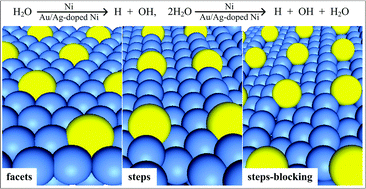
Water–metal interaction has been receiving extensive attention due to its interdisciplinary application. In this paper, on the basis of first-principle calculations and slab models, the behavior of water adsorption and dissociation on planar, stepped and blocked Ni surfaces is investigated, the effects of steps, Au and Ag dopants, coverage and self-aggregation of water are also disclosed. The results show that: step not only strengthens water–substrate interaction but also facilitates water dissociation. With dopants modification, the adsorption and dissociation properties remain relatively unchanged at lower coverage (1/9 on facets and 1/12 ML on steps) while at higher coverage (1/4 on facets and 1/6 ML on steps) water adsorption is weakened and dissociation activity decreases dramatically. Water adsorption and dissociation properties on Ni surfaces are essentially unaffected with the increase of coverage. On doped surfaces, adsorption properties and dissociation activities are closely associated with the ligand effect, which is dependent on the dopant, dopant concentration and surface morphology. Water self-aggregation enhances water–surface interaction on all studied surfaces due to hydrogen bond (network) formation. Furthermore, investigation shows that it does not assist water dissociation.

 Please wait while we load your content...
Please wait while we load your content...