Influence of natural adsorbates of magnesium oxide on its reactivity in basic catalysis
Abstract
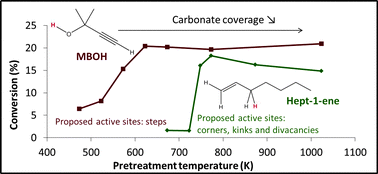
Solid materials possessing basic properties are naturally covered by carbonates and hydroxyl groups. Those natural adsorbates modify their chemical reactivity. This article aims to specifically evidence the role of surface carbonates and hydroxyls in basic heterogeneous catalysis on MgO. It compares the catalytic behaviors of hydroxylated or carbonated MgO surfaces for two types of reactions: one alkene isomerization and one alcohol conversion (hept-1-ene isomerization and 2-methyl-3-butyn-2-ol conversion). Catalysis experiments showed that carbon dioxide adsorption poisons the catalyst surface and the DRIFT–DFT combination showed that the nature of active sites in the two reactions differs. On the reverse, partial hydroxylation of the surface enhances activity for both reactions. Interestingly hept-1-ene isomerization gives a volcano curve for the conversion as a function of hydroxyl coverage. Calculations of the electronic structure of magnesium oxide surfaces show that neither Lewis basicity nor Brønsted basicity of the surface defects (steps for example) are enhanced by hydroxylation. Meanwhile CO2 adsorption followed by IR spectroscopy shows that (110) and (111) unstable planes are strongly basic and are stabilized by partial surface hydroxylation. These results could explain the volcano curve obtained for the evolution of alkene isomerisation as a function of hydroxyl coverage.

 Please wait while we load your content...
Please wait while we load your content...