Dynamics of benzimidazole ethylphosphonate: a solid-state NMR study of anhydrous composite proton-conducting electrolytes†
Abstract
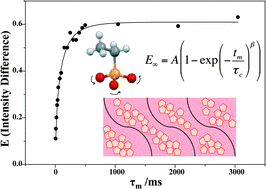
Imidazole phosphate and phosphonate solid acids model the hydrogen-bonding networks and dynamics of the anhydrous electrolyte candidate for proton exchange membrane fuel cells. Solid-state NMR reveals that phosphate and phosphonate anion dynamics dominate the rate of long-range proton transport, and that the presence of a membrane host facilitates proton mobility, as evidenced by a decreased correlation time of the composites (80 ± 15 ms) relative to the pristine salt (101 ± 5 ms). Benzimidazole ethylphosphonate (Bi-ePA) is chosen as a model salt to investigate the membrane system. The hydrogen-bonding structure of Bi-ePA is established using X-ray diffraction coupled with solid-state 1H–1H DQC NMR. The anion dynamics has been determined using solid-state 31P CODEX NMR. By comparing the dynamics of ethylphosphonate groups in pristine salt and membrane–salt composites, it is clear that the rotation process involves three-site exchange. Through data interpretation, a stretched exponential function is introduced with the stretching exponent, β, ranging 0 < β ≤ 1. The 31P CODEX data for pristine salt are fitted with single exponential decay where β = 1; however, the data for the membrane–salt composites are fitted with stretched exponential functions, where β has a constant value of 0.5. This β value suggests a non-Gaussian distribution of the dynamic systems in the composite sample, which is introduced by the membrane host.

 Please wait while we load your content...
Please wait while we load your content...