Unified approach to multipolar polarisation and charge transfer for ions: microhydrated Na+†
Abstract
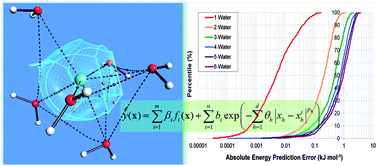
Electrostatic effects play a large part in determining the properties of chemical systems. In addition, a treatment of the polarisation of the electron distribution is important for many systems, including solutions of monatomic ions. Typically employed methods for describing polarisable electrostatics use a number of approximations, including atom-centred point charges and polarisation methods that require iterative calculation on the fly. We present a method that treats charge transfer and polarisation on an equal footing. Atom-centred multipole moments describe the charge distribution of a chemical system. The variation of these multipole moments with the geometry of the surrounding atoms is captured by the machine learning method kriging. The interatomic electrostatic interaction can be computed using the resulting predicted multipole moments. This allows the treatment of both intra- and interatomic polarisation with the same method. The proposed method does not return explicit polarisabilities but instead, predicts the result of the polarisation process. An application of this new method to the sodium cation in a water environment is described. The performance of the method is assessed by comparison of its predictions of atomic multipole moments and atom–atom electrostatic interaction energies to exact results. The kriging models are able to predict the electrostatic interaction energy between the ion and all water atoms within 4 kJ mol−1 for any of the external test set Na+(H2O)6 configurations.

 Please wait while we load your content...
Please wait while we load your content...