The reaction of formaldehyde carbonyl oxide with the methyl peroxy radical and its relevance in the chemistry of the atmosphere†
Abstract
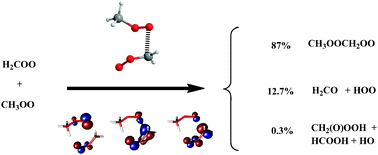
The reaction of formaldehyde carbonyl oxide (H2COO) with the methyl peroxy radical (CH3OO), a prototype of the reactions of carbonyl oxides with alkyl peroxy radicals of potential interest in atmospheric chemistry, has been investigated by means of quantum-mechanical electronic structure methods (CASSCF, CASPT2, UQCISD, and UCCSD(T)) and DFT functionals (B3LYP, BH&HLYP, M05 and M06-2X). Two reaction paths have been found for the lowest-barrier reaction, namely the CH3OO radical addition to the carbon atom of H2COO leading to the formation of the CH3OOCH2OO radical adduct. Both pathways begin with the formation of a pre-reactive complex with binding energies of 5.39 and 5.13 kcal mol−1. The corresponding transition states are predicted to lie 2.64 and 0.25 kcal mol−1, respectively, below the energy of the reactants and the rate constant of the global reaction is calculated to be 3.74 × 10−12 cm3 molecules−1 s−1 at 298 K. Since the CH3OOCH2OO radical adduct is formed with an internal energy excess of about 45 kcal mol−1, it can decompose unimolecularly into formaldehyde and the CH2(O)OOCH radical. This unimolecular decomposition involves an intramolecular H-atom transfer followed by the decomposition of the CH2OOCH2OOH radical intermediate. Kinetic calculations based on the collision–reaction master equation employing the MultiWell Program Suite reveal that the CH3OOCH2OO radical adduct is stabilized in 86.9%, whereas 13.10% of the reaction corresponds to the formation of H2CO plus the CH2(O)OOH radical. It is concluded that the methyl peroxy radical addition to substituted carbonyl oxides might be the source of low volatility oligomers observed in secondary organic aerosols in chamber studies.

 Please wait while we load your content...
Please wait while we load your content...