Production of gas phase NO2 and halogens from the photolysis of thin water films containing nitrate, chloride and bromide ions at room temperature†
Abstract
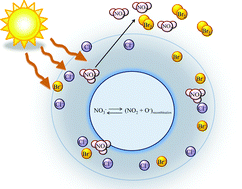
Nitrate and halide ions coexist in particles generated in marine regions, around alkaline dry lakes, and in the Arctic snowpack. Although the photochemistry of nitrate ions in bulk aqueous solution is well known, there is recent evidence that it may be more efficient at liquid–gas interfaces, and that the presence of other ions in solution may enhance interfacial reactivity. This study examines the 311 nm photolysis of thin aqueous films of ternary halide–nitrate salt mixtures (NaCl–NaBr–NaNO3) deposited on the walls of a Teflon chamber at 298 K. The films were generated by nebulizing aqueous 0.25 M NaNO3 solutions which had NaCl and NaBr added to vary the mole fraction of halide ions. Molar ratios of chloride to bromide ions were chosen to be 0.25, 1.0, or 4.0. The subsequent generation of gas phase NO2 and reactive halogen gases (Br2, BrCl and Cl2) were monitored with time. The rate of gas phase NO2 formation was shown to be enhanced by the addition of the halide ions to thin films containing only aqueous NaNO3. At [Cl−]/[Br−] ≤ 1.0, the NO2 enhancement was similar to that observed for binary NaBr–NaNO3 mixtures, while with excess chloride NO2 enhancement was similar to that observed for binary NaCl–NaNO3 mixtures. Molecular dynamics simulations predict that the halide ions draw nitrate ions closer to the interface where a less complete solvent shell allows more efficient escape of NO2 to the gas phase, and that bromide ions are more effective in bringing nitrate ions closer to the surface. The combination of theory and experiments suggests that under atmospheric conditions where nitrate ion photochemistry plays a role, the impact of other species such as halide ions should be taken into account in predicting the impacts of nitrate ion photochemistry.

 Please wait while we load your content...
Please wait while we load your content...