Nitrogen-enriched carbon electrodes in electrochemical capacitors: investigating accessible porosity using CM-SANS†
Abstract
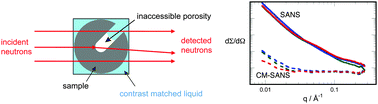
Carbon electrochemical capacitor electrodes containing nitrogen groups were studied with respect to their electrochemical behaviour, chemical composition and physical characteristics. Thermal treatment of

 Please wait while we load your content...
Please wait while we load your content...