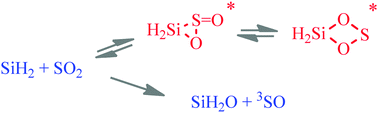
Time-resolved kinetic studies of the reaction of silylene, SiH2, with SO2 have been carried out in the gas phase over the temperature range 297–609 K, using laser flash photolysis to generate and monitor SiH2. The second order rate coefficients at 1.3 kPa (SF6 bath gas) fitted the Arrhenius equation: log(k/cm3 molecule−1 s−1) = (−10.10 ± 0.06) + (3.46 ± 0.45 kJ mol−1)/RT ln 10 where the uncertainties are single standard deviations. The collisional efficiency is 71% at 298 K, and in kinetic terms the reaction most resembles those of SiH2 with CH3CHO and (CH3)2CO. Quantum chemical calculations at the G3 level suggest a mechanism occurring via addition of SiH2 to one of the S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bonds leading to formation of the three-membered ring, thione-siloxirane which has a low energy barrier to ring expansion to yield the four-membered ring, 3-thia-2,4-dioxasiletane, the lowest energy adduct found on the potential energy (PE) surface. RRKM calculations, however, show that, if formed, this molecule would only be partially stabilised under the reaction conditions and the rate coefficients would be pressure dependent, in contrast with experimental findings. The G3 calculations reveal the complexity of possible intermediates and end products and taken together with the RRKM calculations indicate the most likely end products to be H2SiO + SO (3Σ−). The reaction is compared and contrasted with that of SiH2 + CO2.
O double bonds leading to formation of the three-membered ring, thione-siloxirane which has a low energy barrier to ring expansion to yield the four-membered ring, 3-thia-2,4-dioxasiletane, the lowest energy adduct found on the potential energy (PE) surface. RRKM calculations, however, show that, if formed, this molecule would only be partially stabilised under the reaction conditions and the rate coefficients would be pressure dependent, in contrast with experimental findings. The G3 calculations reveal the complexity of possible intermediates and end products and taken together with the RRKM calculations indicate the most likely end products to be H2SiO + SO (3Σ−). The reaction is compared and contrasted with that of SiH2 + CO2.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O double bonds leading to formation of the three-membered ring, thione-siloxirane which has a low energy barrier to
O double bonds leading to formation of the three-membered ring, thione-siloxirane which has a low energy barrier to 

 Please wait while we load your content...
Please wait while we load your content...