Molecular structure and hydrogen bonding in pure liquid ethylene glycol and ethylene glycol–water mixtures studied using NIR spectroscopy
Abstract
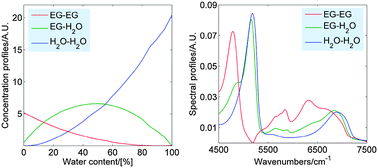
The molecular structure and hydrogen bonding of ethylene glycol (EG) and EG–water mixtures in the liquid phase were studied by using near-infrared (NIR) spectroscopy. The spectra were evaluated using a two-dimensional (2D) correlation approach, moving-window 2D correlation analysis and chemometric methods. The minor changes for the CH stretching bands indicate that the structures of pure liquid EG and EG–water mixtures are determined by the intermolecular hydrogen bonding through the OH groups. The analysis of the ν2 + ν3 combination band of water reveals that in EG-rich solutions the molecules of water are predominantly bonded with two molecules of EG and this cooperative hydrogen bonding is stronger than that in bulk water. Further increase in the water content leads to formation of small water clusters around OH groups of EG. Comparing results for the binary mixtures of water with different organic solvents one can conclude that the total amount and distribution of the polar groups are the most important factors determining the solubility of water in the organic phase. The distribution of these groups depends on the length and structure of the hydrocarbon chain. Due to high population and relatively uniform distribution of the OH groups of EG water has unlimited solubility in liquid EG.

 Please wait while we load your content...
Please wait while we load your content...