Entropies of defect formation in ceria from first principles
Abstract
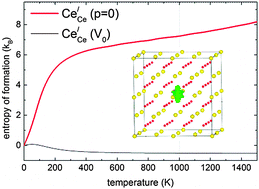
We calculate entropies of formation for fully charged point defects, including the small polaron 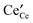 , in undoped fluorite-structured ceria by means of density functional theory in the GGA + U approximation. We discuss the behaviour of the entropy for the constant volume and the constant pressure case. Our results for constant pressure (p = 0) suggest that the change in volume, due to the formation of defects, dominates the entropy of formation. From the individual entropies of formation the entropies of Frenkel, anti-Frenkel and Schottky disorder as well as the entropy of
, in undoped fluorite-structured ceria by means of density functional theory in the GGA + U approximation. We discuss the behaviour of the entropy for the constant volume and the constant pressure case. Our results for constant pressure (p = 0) suggest that the change in volume, due to the formation of defects, dominates the entropy of formation. From the individual entropies of formation the entropies of Frenkel, anti-Frenkel and Schottky disorder as well as the entropy of

 Please wait while we load your content...
Please wait while we load your content...