Superoxide generation from the reduction of oxygen at the carbon–oil–water triple phase boundary
Abstract
The reduction of oxygen is studied in aqueous solutions of pH 6.22–8.01, at a carbon paste electrode fabricated from dioctyl phthalate (oil) and graphite. Two two-electron voltammetric waves are usually seen on carbon electrodes, associated with the formation of hydrogen peroxide and water, respectively. However, an additional signal is seen on the carbon paste electrode, which can attributed to the initial formation of the superoxide radical anion, O2˙−. Data is presented to show that the predominant source of oxygen for this reaction is that dissolved in the carbon paste material, rather than in the aqueous solution, and that the superoxide is likely formed at the graphite–oil–water triple phase boundary. Kinetic and thermodynamic parameters for the O2/O2˙− redox couple are reported.
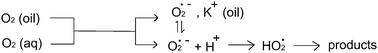

 Please wait while we load your content...
Please wait while we load your content...