First-principles melting of gallium clusters down to nine atoms: structural and electronic contributions to melting
Abstract
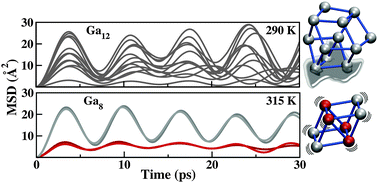
First-principles Born–Oppenheimer molecular dynamics simulations of small gallium clusters, including parallel tempering, probe the distinction between cluster and molecule in the size range of 7–12 atoms. In contrast to the larger sizes, dynamic measures of structural change at finite temperature demonstrate that Ga7 and Ga8 do not melt, suggesting a size limit to melting in gallium exists at 9 atoms. Analysis of electronic structure further supports this size limit, additionally demonstrating that a covalent nature cannot be identified for clusters larger than the gallium dimer. Ga9, Ga10 and Ga11 melt at greater-than-bulk temperatures, with no evident covalent character. As Ga12 represents the first small gallium cluster to melt at a lower-than-bulk temperature, we examine the structural properties of each cluster at finite temperature in order to probe both the origins of greater-than-bulk melting, as well as the significant differences in melting temperatures induced by a single atom addition. Size-sensitive melting temperatures can be explained by both energetic and entropic differences between the solid and liquid phases for each cluster. We show that the lower-than-bulk melting temperature of the 12-atom cluster can be attributed to persistent pair bonding, reminiscent of the pairing observed in α-gallium. This result supports the attribution of greater-than-bulk melting in gallium clusters to the anomalously low melting temperature of the bulk, due to its dimeric structure.

 Please wait while we load your content...
Please wait while we load your content...