The dissociation of glycolate—astrochemical and prebiotic relevance†
Abstract
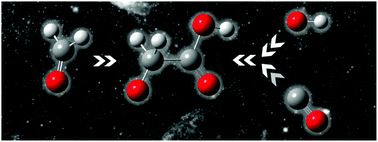
On the basis of mass spectrometric experiments and quantum chemical calculations, including detailed kinetic and dynamics calculations, we report the unimolecular dissociation of an isolated glycolate anion. The dominating processes are: loss of formaldehyde; loss of carbon monoxide; loss of carbon dioxide; and loss of a hydrogen molecule, with the latter having the lowest energetic threshold. At higher energies, CO loss is the dominating reaction. The loss of CO may be followed by a second CO loss, leading to the H−H2O complex in close mechanistic relationship to the Nibbering reaction. The results provide valuable insights into possible mechanisms for interstellar and prebiotic formation of glycolate via the reverse of the unimolecular dissociation reactions. We propose that the addition of the complex of OH− and CO to CH2O is the most feasible route to gas phase synthesis of glycolate, since all species are abundant in interstellar space.

 Please wait while we load your content...
Please wait while we load your content...