Enhanced hydrogen formation during the catalytic decomposition of H2O2 on metal oxide surfaces in the presence of HO radical scavengers
Abstract
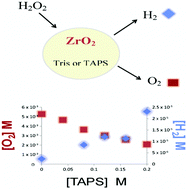
Presently and for the foreseeable future, hydrogen peroxide and transition metal oxides are important constituents of energy production processes. In this work, the effect of the presence of HO radical scavengers on the product yield from the decomposition of H2O2 on metal oxide surfaces in aqueous solution was examined experimentally. Scavenging the intermediate product HO˙ by means of Tris or TAPS buffer leads to enhanced formation of H2. In parallel, a decrease in the production of the main gaseous product O2 is observed. Under these conditions, H2 formation is a spontaneous process even at room temperature. The yields of both the H2 and O2 depend on the concentration of Tris or TAPS in the reaction media. We observed that TAPS has a higher affinity for the surface of ZrO2 than does Tris. The difference in adsorption of both scavengers is reflected by the difference in their influence on the product yields. The observed sensitivity of the system H2O2–ZrO2 towards the two different scavengers indicates that O2 and H2 are formed at different types of surface sites.

 Please wait while we load your content...
Please wait while we load your content...