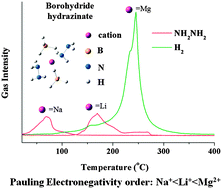
Four new borohydride hydrazinates, including NaBH4·NH2NH2, LiBH4·1/2NH2NH2, LiBH4·1/3NH2NH2 and Mg(BH4)2·3NH2NH2, were synthesized. NaBH4·NH2NH2 and Mg(BH4)2·3NH2NH2 possess monoclinic and trigonal structures, respectively, while LiBH4·1/2NH2NH2 and LiBH4·1/3NH2NH2 exhibit orthorhombic and monoclinic structures. The effects of composition on the dehydrogenation of hydrazinates were investigated. It is demonstrated that cations with high Pauling electronegativity hold hydrazine strongly in the vicinity of borohydride and result in direct dehydrogenation at elevated temperatures. Specifically, Mg(BH4)2 hydrazinates can directly generate hydrogen upon heating it under a flow of Ar; on the other hand, the Li and Na counterparts lost part or all of the hydrazine components under the same condition. In addition, reducing NH2NH2 content in the complexes leads to improved dehydrogenation properties. Mechanistic investigation of Mg(BH4)2 hydrazinates using isotopic labelling indicates that hydrogen desorption is via homogeneous dissociation of N–N bond of NH2NH2 followed by the establishment of B–N bond and combination of Hδ+ (N) and Hδ− (B).
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...