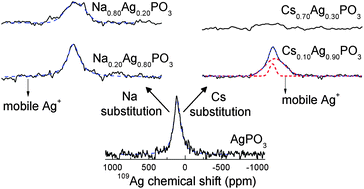
Metaphosphate glasses with two monovalent species A1−xBxPO3 (0 ≤ x ≤ 1) show mixed-ion effects (MIE) in the dc conductivities and glass transition temperatures, which are strongly dependent on the cation size mismatch between the two mobile species. In the present contribution, mixed-ion metaphosphate glasses based on the cation combinations Cs–Li, Rb–Li, and Cs–Ag, exhibiting particularly large size mismatches, are analyzed by 31P, 87Rb, 109Ag and 133Cs NMR to determine possible correlations between this mismatch and some of the structural properties critical to the development of the MIE: the local environments around the mobile species and their spatial distribution relative to each other. The results are compared with those obtained in the Na–Ag metaphosphate series, which serves as a reference system, with minimized cation mismatch MIE. The local coordination environments of the Ag+, Rb+ and Cs+ ions follow analogous compositional trends as previously observed in Na-based mixed-ion metaphosphate glasses: for a given cation species A, the average A–O distance shows an expansion/compression when this cation is replaced by a second species B with smaller/bigger ionic radius, respectively. This compositional differentiation of the structural sites for the mobile species may contribute to the MIE. Concerning the relative spatial distribution of the mobile ions, results from 7Li–133Cs (SEDOR) experiments indicate a random mixture of Cs and Li in Cs–Li metaphosphate glasses. While this result is in agreement with one of the fundamental hypotheses of the models proposed to describe the MIE, it is at variance with the observation of various partial cation segregation phenomena observed in Na-based mixed alkali glasses. This result suggests that cation size mismatch is not the decisive parameter in determining segregation or non-statistical mixing of cations in the glass. In the Cs–Ag and Na–Ag glasses, 109Ag spin-echo NMR reveals a progressive slowing down of the Ag+ diffusion dynamics as this species is replaced by Cs+ or Na+. The substitution by the bigger Cs+ ion causes a strong reduction in Ag+ mobility suggesting the existence of separated diffusion pathways for these cations. In contrast, substitution by the similarly-sized Na+ causes a much weaker mobility reduction consistent with the existence of Ag–Na cooperative hopping.

 Please wait while we load your content...
Please wait while we load your content...