Methodological keys for accurate
 simulations†
simulations†
Abstract
Photoacids have a stronger propensity to give 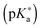 remains challenging as the lifetime of the photoacid, in its excited state, is too small. The present article establishes several protocols using the latest developments of the PCM-TD-DFT formalism e.g. both the corrected linear response (cLR) and state specific (SS) approaches. Equilibrium (eq) and non-equilibrium (neq) limits of the implicit
remains challenging as the lifetime of the photoacid, in its excited state, is too small. The present article establishes several protocols using the latest developments of the PCM-TD-DFT formalism e.g. both the corrected linear response (cLR) and state specific (SS) approaches. Equilibrium (eq) and non-equilibrium (neq) limits of the implicit



 Please wait while we load your content...
Please wait while we load your content...
 simulations
simulations