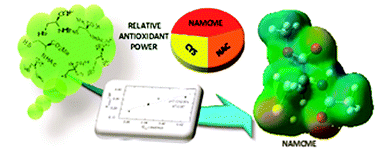
The challenge of developing organic molecules with improved antioxidant activities for a competitive marketplace requires, given the great amount of possibilities, much laboratory work. Nowadays, the ability of methodologies based on quantum chemistry to determine the influence of different modifications on a molecule core provides a powerful tool for selecting the most useful derivatives to be synthesized. Here, we report the results of the assessment of antioxidant activity for quaternary amino acids, specifically for cysteine derivatives. The effect of introducing different substituents on the cysteine core is evaluated by using DFT to obtain an adequate structure–antioxidant activity relationship. This theoretical study shows a small panel of targets among which (R)-N-acetyl-2-methylcysteine methyl ester 15 exhibits special features and relevant antioxidant activity. The conformational 1H NMR study of this synthesized compound indicates the existence of an intramolecular C7 member ring involving S–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C substructure, which is reported for the first time in the literature for this amino acid unit. This unusual conformation seems to be the reason for the high antioxidant capacity experimentally found for this compound.
C substructure, which is reported for the first time in the literature for this amino acid unit. This unusual conformation seems to be the reason for the high antioxidant capacity experimentally found for this compound.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C substructure, which is reported for the first time in the literature for this amino acid unit. This unusual conformation seems to be the reason for the high
C substructure, which is reported for the first time in the literature for this amino acid unit. This unusual conformation seems to be the reason for the high 

 Please wait while we load your content...
Please wait while we load your content...