Effects of convergent diffusion and charge transfer kinetics on the diffusion layer thickness of spherical micro- and nanoelectrodes
Abstract
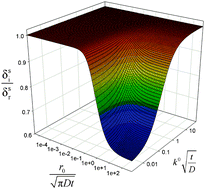
Nuances of the linear diffusion layer approximation are examined for slow charge transfer reactions at (hemi)spherical micro- and nanoelectrodes. This approximation is widely employed in Electrochemistry to evaluate the extent of electrolyte solution perturbed by the electrode process, which is essential to the understanding of the effects arising from thin-layer diffusion, convergent diffusion, convection, coupled chemical reactions and the double layer. The concept was well established for fast charge transfer processes at macroelectrodes, but remains unclear under other conditions such that a thorough assessment of its meaning was necessary. In a previous publication [A. Molina, J. González, E. Laborda and R. G. Compton, Phys. Chem. Chem. Phys., 2013, 15, 2381–2388] we shed some light on the influence of the reversibility degree. In the present work, the meaning of the diffusion layer thickness is investigated when very small electrodes are employed and so the contribution of convergent diffusion to the mass transport is very important. An analytical expression is given to calculate the linear diffusion layer thickness at (hemi)spherical electrodes and its behaviour is studied for a wide range of conditions of reversibility (from reversible to fully-irreversible processes) and

 Please wait while we load your content...
Please wait while we load your content...