Axial iron coordination and spin state change in a hemec upon electrostatic protein–SAM interaction†
Abstract
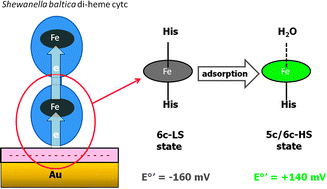
A bacterial di-heme cytochrome c binds electrostatically to a gold electrode surface coated with a negatively charged COOH-terminated SAM adopting a sort of ‘perpendicular’ orientation.

 Please wait while we load your content...
Please wait while we load your content...