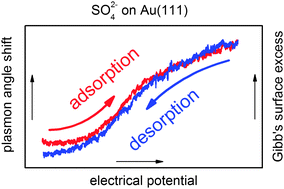
In situ determination of the surface excess upon electrochemical sulfate adsorption on Au(111) films by surface plasmon resonance
Abstract
For the independent and simultaneous characterization of electrochemical processes, we employ
- This article is part of the themed collection: Bunsentagung 2013: 'Theory meets Spectroscopy'

 Please wait while we load your content...
Please wait while we load your content...