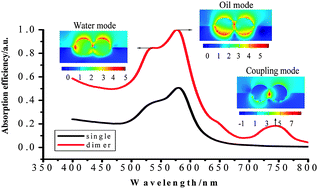
Unlike the solid–air and solid–liquid interfaces, the optical properties of metal nanoparticles adsorbed at the liquid–liquid interface have not been theoretically exploited to date. In this work, the three dimensional finite difference time domain (3D-FDTD) method is employed to clarify the localized surface plasmon resonance (LSPR) based optical properties of gold nanoparticles (NPs) adsorbed at the water–oil interface, including near field distribution, far field absorption and their relevance. The LSPR spectra of NPs located at a liquid–liquid interface are shown to differ significantly from those in a uniform liquid environment or at the other interfaces. The absorption spectra exhibit two distinct LSPR peaks, the positions and relative strengths of which are sensitive to the dielectric properties of each liquid and the exact positions of the NPs with respect to the interface. Precise control of the particles’ position and selection of the appropriate wavelength of the excitation laser facilitates the rational design and selective excitation of localized plasmon modes for interfacial NPs, a necessary advance for the exploration of liquid–liquid interfaces via surface enhanced Raman spectroscopy (SERS). According to our calculations, the SERS enhancement factor for Au nanosphere dimers at the water–oil interface can be as high as 107–109, implying significant promise for future investigations of interfacial structure and applications of liquid–liquid interfaces towards chemical analysis.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...