The role of the hydrogen bond in dense nanoparticle–gas suspensions
Abstract
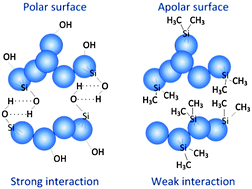
The effect of surface characteristics on the interaction between
- This article is part of the themed collection: Rising Stars and Young Nanoarchitects in Materials Science

 Please wait while we load your content...
Please wait while we load your content...