Theoretical investigations into the nucleation of silica growth in basic solution part I – ab Initio studies of the formation of trimers and tetramers†
Abstract
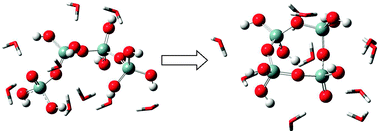
We have performed the first ab initio study that considers all possible oligomerization reactions available to

 Please wait while we load your content...
Please wait while we load your content...