First principles study on the hydrophilic and conductive graphene doped with Al atoms
Abstract
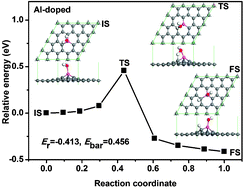
The effect of the Al dopant on the dissociative adsorption of a H2O molecule on graphene is investigated using first principles calculations. It is found that doping Al into graphene can facilitate the dissociative adsorption of H2O molecules. The dissociative energy barrier is reduced from 3.609 eV on pristine graphene to 0.456 eV on Al-doped graphene and the reaction releases an energy of 0.413 eV, which indicates a smooth dissociative adsorption on Al-doped graphene at room temperature. In addition, the dissociative adsorption of H2O molecules can convert the Al-doped graphene from hydrophobic to hydrophilic while obtaining conductive graphene with doping concentration higher than 5.56%. This hydrophilic and conductive graphene has potential applications in supercapacitors and biomaterial supports.

 Please wait while we load your content...
Please wait while we load your content...