Formation and decay of a compressed phase of 4,4′-biphenyldicarboxylic acid on Cu(001)
Abstract
The molecular arrangement of 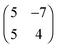 superstructure in matrix notation. All four equivalent (rotational and mirror) domains are equally populated. Both the c(8 × 8) and the compressed phase are confined to the first layer and the latter has a 14% higher density compared to the c(8 × 8) phase. Remarkably, this compressed phase is stable only during deposition and decays after interruption of the deposition. Apparently, the density of physisorbed admolecules on top of the c(8 × 8) layer has to be above a relevant threshold to allow the formation of the compressed phase.
superstructure in matrix notation. All four equivalent (rotational and mirror) domains are equally populated. Both the c(8 × 8) and the compressed phase are confined to the first layer and the latter has a 14% higher density compared to the c(8 × 8) phase. Remarkably, this compressed phase is stable only during deposition and decays after interruption of the deposition. Apparently, the density of physisorbed admolecules on top of the c(8 × 8) layer has to be above a relevant threshold to allow the formation of the compressed phase.


 Please wait while we load your content...
Please wait while we load your content...