The strain effect on colossal oxygen ionic conductivity in nanoscale zirconia electrolytes: a first-principles-based study
Abstract
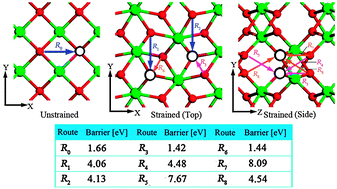
Density functional theory calculations and first-principles molecular dynamics (MD) simulations have been performed to examine the strain effect on the colossal oxygen ionic conductivity in selected sandwich structures of

 Please wait while we load your content...
Please wait while we load your content...