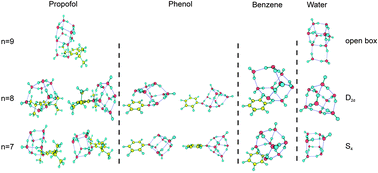
Following previous structural investigations on the hydrated clusters of the anesthetic propofol we analyze here the spectroscopy of propofol·(H2O)7–9 in supersonic expansions, to approach to the solution limit. Using 2-color REMPI the mass-resolved electronic spectrum of each solvated species was obtained. The UV/UV hole burning demonstrated the presence of at least two conformers for propofol·(H2O)7 and propofol·(H2O)8, while a single conformer was observed for propofol·(H2O)9. Structural information from each isomer was obtained using IR/UV hole burning, both in the mid-IR and in the OH region. Comparison of vibrational data with those from calculations at M06-2X/6-311++G(d,p) demonstrates that the water molecules form polyhedral structures that resemble those found in pure water clusters. Comparison with the results obtained for the hydrated clusters of phenol and benzene shows that similar structures are formed, although some relevant differences are found, mainly in the number of isomers present for each stoichiometry.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...