Formation of high crystalline ZIF-8 in an aqueous solution†
Abstract
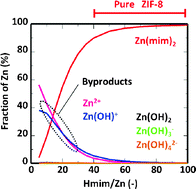
We report here the synthesis of a zeolitic imidazolate framework-8 (ZIF-8) in an aqueous solution. ZIF-8 crystals were prepared by mixing 2-methylimidazole (Hmim) with Zn nitrate hexahydrate (Zn) in deionized water. The products prepared at high Hmim/Zn molar ratios were assigned to a sodalite (SOD)-type structure whose morphology consisted of a rhombic dodecahedron with truncated corners. The crystals possessed ultrahigh surface areas and micropore volumes. At low Hmim/Zn molar ratio, some zinc hydroxide and basic zinc nitrate were observed in the products. We also focused on the formation process of ZIF-8 crystals in an aqueous system by observing the change in the pH value as a function of synthesis time. We tried to calculate the stability constant of ZIF-8 by fitting the calculated pH values to the measured pH values. When the molar fractions of the zinc compounds in the equilibrium state were calculated, high fractions of the Zn(mim)2 complex were observed at high concentrations of Hmim. On the other hand, some zinc cations were present at low concentrations of Hmim. This finding would support a causal relationship between these zinc cations and the formation of some by-products.
- This article is part of the themed collection: A celebration of 25 volumes of CrystEngComm

 Please wait while we load your content...
Please wait while we load your content...