Crystal structures of discrete, one-dimensional and cocrystalline copper(ii)–uranyl(vi) systems: the influence of the reactant ratio in the competition between hydrogen bonds and coordinate bonds†
Abstract
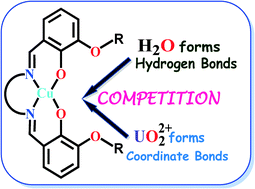
This paper presents the syntheses and crystal structures of four copper(II)–uranyl(VI) compounds [{CuIILOEt–en}2·{(UVIO2)(NO3)2(H2O)2}] (1), [CuII(MeCN)LOEt–pn(UVIO2)(NO3)2] (2), [(UVIO2)2(μ-H2O)2(NO3)4]∙4[CuIILOEt–py(H2O)]∙2MeCN (3) and {[CuIILOMe–en(UVIO2)(NO3)]2[(UVIO2)2(μ-HO)2(NO3)4]}n (4), where H2LOEt–en, H2LOEt–pn, H2LOEt–py and H2LOMe–en are 3-ethoxysalicylaldehyde-diamine (H2LOEt) or 3-methoxysalicylaldehyde-diamine (H2LOMe) ligands in which the diamine functionality are ethylenediamine, 1,3-diaminopropane, 2,3-diaminopyridine and ethylenediamine, respectively. Compound 1 is a [1 × 2 + 1 × 1] trinuclear cocrystal containing two mononuclear [CuIILOEt–en] and one mononuclear [(UVIO2)(NO3)2(H2O)2] moiety; two coordinated water molecules interact with two O4 compartments by forming hydrogen bonds. Compound 2 is a dinuclear system in which two phenoxo oxygen atoms coordinate with the uranium(VI) centre. Compound 3 is a [2 × 1 + 1 × 4] hexanuclear cocrystal of four mononuclear [CuIILOEt–py(H2O)] units and one dinuclear [(UVIO2)2(μ-H2O)2(NO3)4] moiety; O–H⋯O and C–H⋯O interactions interlink the five units here. In 4, there are two dinuclear [CuIILOMe–en(UVIO2)(NO3)]+ cations and one dinuclear [(UVIO2)2(μ-HO)2(NO3)4]2− anion, which are self-assembled to generate a one-dimensional topology. Interesting structural aspects including the weak interaction directed self-assemblies and most importantly, the competition between hydrogen bond and coordinate bond formation and the influence of the reactant ratio in governing that competition are discussed. The diffuse reflectance or solution transmission spectra (for the d–d band) of 1–4 and the corresponding mononuclear copper(II) precursors are also described.

 Please wait while we load your content...
Please wait while we load your content...