The competition between halogen bonds (Br⋯O) and C–H⋯O hydrogen bonds: the structure of the acetone–bromine complex revisited†
Abstract
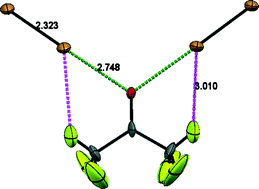
A combination of single-crystal X-ray (at 110 and 200 K) and high-resolution neutron powder diffraction (110 K only) using perdeuterated samples has been used to re-determine the crystal structure of the well-known acetone–Br2 complex. The results indicate a significant interaction between the carbonyl oxygen and the nearest Br atom leading to a marked elongation of the molecular Br–Br bond. In the earlier widely cited crystal structure, no such elongation was reported. Furthermore, the new structure indicates the presence of additional weaker C–H⋯O and C–H⋯Br interactions. In this simple complex, in which the halogen bonding is strong compared to the hydrogen bonding (C–H⋯O), the former interaction is found to occur close (~12°) to the plane of the carbonyl group, with the C–H⋯Br complementing the stronger halogen bond.

 Please wait while we load your content...
Please wait while we load your content...