1 : 1 and 2 : 1 co-crystals of alkoxystilbazoles with tetrafluoroiodobenzenes: halogen bonding, a rare Carene–H⋯N hydrogen bond and unsymmetric iodine⋯pyridine interactions†
Abstract
The single crystal structure of five halogen-bonded co-crystals are reported, formed between para-hydrotetrafluoroiodobenzene and C4, C6, C8 and C10 stilbazoles as well as between C6 stilbazole and ortho- and meta-hydrotetrafluoroiodobenzene. Five of these co-crystals are formed from a 1 : 1 ratio of stilbazole to iodoaromatic, while in three cases, all with para-hydrotetrafluoroiodobenzene, a 2 : 1 stoichiometry is found. Halogen bond distances are between 2.795(2) and 2.877(3) Å, while the I⋯N–Cipso angle is between 160.22(6) and 174.9(1)°. The packing of these co-crystals is described and is discussed in relation to the efficient filling of space, leading to the conclusion that the halogen bond will adopt a non-ideal disposition to facilitate better solid-state packing. It is also shown that steric interactions between alkyl hydrogens and aromatic fluorines contribute to the determination of packing efficiency. Unexpectedly, in the case of the C6 stilbazole complexed by para-hydrotetrafluoroiodobenzene, there is a C–H⋯N hydrogen bond with an N⋯H separation of 2.273(2) Å. Non-linear halogen bonds lead to an unsymmetric disposition of the iodine with respect to the pyridyl ring leading to significant differences in non-covalent C⋯I distances, some of which can formally be considered as covalent. This unsymmetric approach of the C–I bond to the pyridyl ring is interpreted in relation to the sum of the forces governing formation of the unit cell.
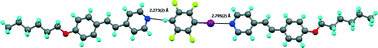

 Please wait while we load your content...
Please wait while we load your content...