Solid state photodimerisation of tetrathiafulvalene derivatives bearing carboxylate and carboxylic acid substituents†
Abstract
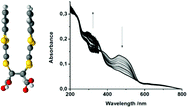
Structural and spectroscopic studies on the [2+2] photodimerisation of TTF derivatives show that solid state cycloaddition leads to a dimer with a face-to-face structure. The type of phototransformation of the material depends not only on the optical characteristics of the reactant but also on those of the product.

 Please wait while we load your content...
Please wait while we load your content...