Influence of chemical and structural factors on the calcite–calcium oxalate transformation
Abstract
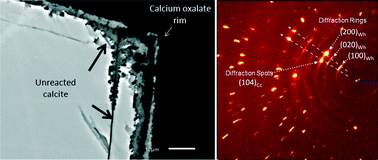
The mechanisms and epitaxial relationships of the replacement of calcite by calcium oxalate have been studied by means of SEM, 2D-XRD and AFM techniques. At acidic pH, oxalate-bearing solutions react with freshly cleaved calcite fragments through a pseudomorphic, coupled dissolution–precipitation reaction. This replacement reaction takes place by the dissolution of the calcite substrate followed by the precipitation of whewellite (CaC2O4·H2O) crystals, which nucleate and grow epitaxially on the (104) calcite surface. There are two sets of preferred orientations of the whewellite crystals (COM) on the calcite surface (Cc): (010)COM∥(104)Cc, [100]COM∥[![[4 with combining macron]](https://www.rsc.org/images/entities/char_0034_0304.gif) 41]Cc, and (100)COM∥(104)Cc, [001]COM∥[010]Cc. These epitaxial relationships can be understood when comparing the crystalline structure of both minerals. Simultaneous evolution of porosity in this system indicates that solute transport at the mineral–fluid interface is the rate-limiting step necessary to achieve spatial coupling between calcite dissolution and calcium oxalate nucleation and growth so that a pseudomorph is obtained. Understanding the mechanisms that control these mineral reactions has far-reaching implications for the understanding of many processes including the design of protective treatments for building stones or the optimization of phosphorus availability in soils. The results of this study may also offer interesting insights into the mechanisms of stone formation in the presence of biological fluids.
41]Cc, and (100)COM∥(104)Cc, [001]COM∥[010]Cc. These epitaxial relationships can be understood when comparing the crystalline structure of both minerals. Simultaneous evolution of porosity in this system indicates that solute transport at the mineral–fluid interface is the rate-limiting step necessary to achieve spatial coupling between calcite dissolution and calcium oxalate nucleation and growth so that a pseudomorph is obtained. Understanding the mechanisms that control these mineral reactions has far-reaching implications for the understanding of many processes including the design of protective treatments for building stones or the optimization of phosphorus availability in soils. The results of this study may also offer interesting insights into the mechanisms of stone formation in the presence of biological fluids.

 Please wait while we load your content...
Please wait while we load your content...