Mechanism and kinetic studies on the synthesis of LiFePO4via solid-state reactions
Abstract
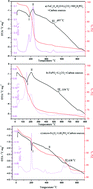
Three classical iron sources including FePO4·2H2O, FeC2O4·2H2O and Fe2O3 are explored to study the mechanism and kinetics of LiFePO4 synthesis via solid-state reactions. During the initial formation of LiFePO4, the reactivity of FePO4 appears to be higher than that of FeC2O4 because the latter involves two stages including the decomposition of FeC2O4 and then the formation of LiFePO4. Fe2O3 shows the lowest reactivity among the solid-state reactions. Meanwhile, the optimal sintering temperature for FePO4 to form LiFePO4 is lower than those for FeC2O4·2H2O and Fe2O3. A larger primary particle size should be responsible for the lower specific capacity of LiFePO4 synthesized with micro-Fe2O3. The kinetic mechanism for obtaining single-phase LiFePO4 corresponds to the diffusion model, calculated by using Coats–Redfern and Doyle equations in which FeC2O4·2H2O and FePO4·2H2O obey 3-dimensional diffusion (cylinder, GB equation) and Fe2O3 obeys 1-dimensional diffusion.

 Please wait while we load your content...
Please wait while we load your content...