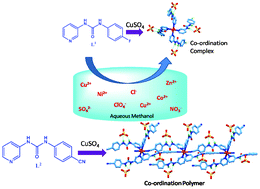
4-Fluorophenyl and 4-cyanophenyl substituted 3-pyridyl urea, L1111 and L2222 respectively, were synthesized and explored extensively to demonstrate SO42− binding via second sphere coordination in their respective self-assembled structures assisted by Cu2+, Cd2+ and Co2+. A single crystal X-ray diffraction study depicts second sphere SO42− recognition in the C4v symmetric cleft formed by the assembly of four molecules of the Cu2+ complex of L1111, [CuL11114(H2O)2]SO4, (1), via eight N–H⋯O interactions from four urea moieties. Similarly, second sphere SO42− recognition via twelve hydrogen bonding interactions (N–H⋯O and C–H⋯O) is also demonstrated in the assembly of the Cd2+ complex of L1111 i.e. [CdL11114(H2O)2]SO4, (2). Detailed structural analysis of 1 and 2 shows the formation of the coordination complexes of Cu2+ and Cd2+ with four units of pyridyl urea, which further assemble to generate a suitable coordination environment for the recognition of SO42− via second sphere coordination. On the other hand, L2222 with a coordinating substituent shows second sphere SO42− coordination with eleven hydrogen bonding interactions (N–H⋯O and C–H⋯O) via the formation of a Cu2+ assisted 1D coordination polymer [{CuL22224}SO4]α, (3). The coordinating property of the –CN of L2222 is reflected in 3, as it directly coordinates to the Cu2+ that assists the formation of the 1D coordination polymer. Second sphere coordination of SO42− in the assembly of L2222 is also established with Co2+ in its complex, [CoL22222(H2O)4]SO4, (4). However, in this case the metal ion prefers to form a coordination complex, as observed in 1 and 2, instead of the 1D-polymeric network in 3. The solution state UV-Vis studies of L1111 with various copper salts, such as Cu(ClO4)2, CuSO4, Cu(NO3)2, CuCl2 and their mixtures show the selective formation of 1. Cu2+ selective second sphere coordination of SO42− in solution is also demonstrated by UV-Vis studies of the complex isolated from the mixtures of various Cu2+ salts or SO42− salts of different metal ions. Furthermore, the selective formation of complex 1 is also demonstrated when complexation of CuSO4 is carried out with mixtures of L1111 and L2222 in MeOH–H2O.

 Please wait while we load your content...
Please wait while we load your content...