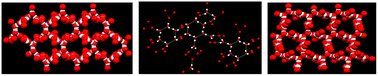
In the three-dimensional (3D) supramolecular assemblies having the general formula [Mn2(dicarboxylate)2(tpa)2]·xH2O (where tpa = N,N,N-tris(2-pyridylmethyl)-amine; dicarboxylate = acetylene dicarboxylate (adc) and x = 6 (1); fumarate and x = 8 (2) and succinate and x = 6 (3)), the dimanganese subunits differing in the dicarboxylate with a variation in the aliphatic chain structure (triple bond to double bond to single bond, respectively) provide the structural diversity of the encapsulated water clusters. These neutral supramolecular assemblies are held together by strong hydrogen bonding and π–π interactions, where the two dimensional (2D) water clusters are formed through strong hydrogen bonding (O–H⋯O). In each case, these water clusters connect the dimanganese subunits through hydrogen bonding with the uncoordinated carboxylate oxygen atoms of the dicarboxylates that bridge the Mn(II) centers of the subunit. The strength of the hydrogen bonding observed in these clusters (the range of O⋯O distances is 2.724 Å to 2.845 Å) is very similar to that found in water and ice. These are synthesized in high yields from a one-pot self-assembly reaction using Mn(OAc)2·4H2O, tpa and the corresponding acid in methanol at ambient conditions. Each of these is characterized by elemental analysis, single crystal and powder X-ray diffraction, IR and Raman spectroscopy and thermogravimetric analysis (TGA). Based on the X-ray crystal structures and TGA, the unusual stability of 2 compared to 1 and 3 is the result of different motifs including a cyclic quasi-planar hexamer formed through hydrogen bonding interactions. In 1 and 2, the Mn(II) centers are hexa-coordinated in a distorted octahedral geometry (N4O2), bonded to four nitrogen atoms of the ligand and two oxygen atoms from two monodentate carboxylate groups, while in 3 each Mn(II) center is heptacoordinated in a pentagonal bipyramidal geometry (N4O3), where in addition to the tpa ligand each succinate binds in chelated as well as monodentate mode, from opposite ends.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...