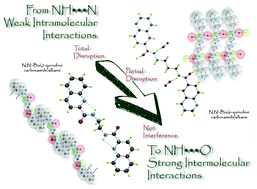
Six new compounds of the homologous series of N,N′-bis(2-quinolinecarboxamide)alkane (4a–c) and N,N′-bis(6-quinolinecarboxamide)alkane (5a–c) derivatives having two, four and six carbon atoms in the alkyl spacer were synthesized and their crystal structures were determined by single crystal X-ray diffraction. These solid-state structures have been analyzed finding that the two series exhibit interesting differences between them. The remarkable preference of the bis(6-quinolinecarboxamide)alkanes to form one- or two-dimensional networks via N–H⋯O intermolecular hydrogen bonds appears total or partially disrupted by the intramolecular N–H⋯Nquinoline hydrogen bond in bis(2-quinolinecarboxamide)alkanes. These interactions, theoretically sorted as a ‘very weak and purely electrostatic’, seems to play a fundamental role in the crystal packing of bis(2-quinolinecarboxamide) derivatives. On the other hand, the rationalization of the bis(6-quinolinecarboxamide) structures are completely in agreement with the statement that the amide-to-amide hydrogen bond formation and network (β-sheets or 4,4-network), in these systems, depends on the interplanar angle between the amide group and the heterocyclic ring.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...