1,6-Bis(1-imidazolyl)-2,4-hexadiyne (1) and 1,6-bis(1-benzimidazolyl)-2,4-hexadiyne (5) have been prepared by a novel method that consists in refluxing excess imidazole and benzimidazole with 2,4-hexadiyne-1,6-diol bis(p-toluenesulfonate), pTS (3). This procedure is a viable alternative to the widely used Hay coupling protocol in case the target diyne possesses substituents capable of deactivating the copper catalyst by complexation. Diyne 1 crystallizes as a hydrate, 1·H2O (2). For this compound, water is essential to achieve a crystalline material, and attempts to obtain crystals without included solvent were unsuccessful. In the structure of 2, the organic fragments organize around the water molecule and interact with it through a dense network of hydrogen bonds. The C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C moieties are not oriented suitably for topochemical polymerization, and when trying to alter the organization of the crystal by heating so as to induce polymerization, water is lost in an abrupt fashion that leads to instantaneous decomposition into polyaromatic-like species. Similar results were observed when water was removed in vacuo at room temperature. The benzimidazole-containing compound can be crystallized with water molecules (4) or without (5). X-ray crystallography shows that the structure of 5 is organized by numerous C–H⋯N, C–H⋯π, and imidazolyl⋯imidazolyl π–π interactions. The diacetylene molecules almost have the right arrangement for topochemical polymerization, with possibly reacting C
C moieties are not oriented suitably for topochemical polymerization, and when trying to alter the organization of the crystal by heating so as to induce polymerization, water is lost in an abrupt fashion that leads to instantaneous decomposition into polyaromatic-like species. Similar results were observed when water was removed in vacuo at room temperature. The benzimidazole-containing compound can be crystallized with water molecules (4) or without (5). X-ray crystallography shows that the structure of 5 is organized by numerous C–H⋯N, C–H⋯π, and imidazolyl⋯imidazolyl π–π interactions. The diacetylene molecules almost have the right arrangement for topochemical polymerization, with possibly reacting C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C fragments not being parallel, a rare situation in diacetylene chemistry. Yet, experiments show that topochemical polymerization does not occur. Incorporation of water in the lattice of 5 leads to a solvate that is topochemically reactive. Unlike 2, however, water molecules in 4 are not isolated but are organized as ribbons. Spectroscopic characterization of the polymer of 4 indicates that it is a blue phase polymer, with water coordinated to it. This study shows that it is possible to use water, and more generally solvent molecules, to transform a nonreactive diacetylene into a reactive one, even though this approach is less predictable than the cocrystal approach developed by Fowler, Lauher, and Goroff. The solvate approach is simple to implement, quite versatile because of the large range of solvents available, and one does not face the problem of having to remove the host in case one needs to recover the polymer. Previous studies describing a similar approach are scarce.
C fragments not being parallel, a rare situation in diacetylene chemistry. Yet, experiments show that topochemical polymerization does not occur. Incorporation of water in the lattice of 5 leads to a solvate that is topochemically reactive. Unlike 2, however, water molecules in 4 are not isolated but are organized as ribbons. Spectroscopic characterization of the polymer of 4 indicates that it is a blue phase polymer, with water coordinated to it. This study shows that it is possible to use water, and more generally solvent molecules, to transform a nonreactive diacetylene into a reactive one, even though this approach is less predictable than the cocrystal approach developed by Fowler, Lauher, and Goroff. The solvate approach is simple to implement, quite versatile because of the large range of solvents available, and one does not face the problem of having to remove the host in case one needs to recover the polymer. Previous studies describing a similar approach are scarce.
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C moieties are not oriented suitably for topochemical
C moieties are not oriented suitably for topochemical ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–C
C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C fragments not being parallel, a rare situation in diacetylene chemistry. Yet, experiments show that topochemical
C fragments not being parallel, a rare situation in diacetylene chemistry. Yet, experiments show that topochemical 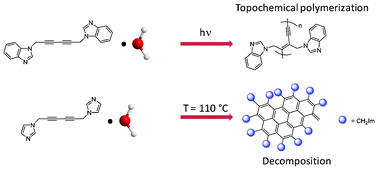

 Please wait while we load your content...
Please wait while we load your content...