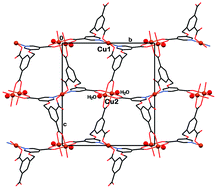
Five new framework solids consisting of coordination polymers [Cd3(L)2(H2O)2(DMA)2(H2O)]n (1), [Cu3(L)2(H2O)2]n (2), [Co3(L)2(H2O)6]n (3), [Mn3(L)2(H2O)5(DMF)]n (4), and [Ni3(L)2(H2O)6]n (5) (H3L = 5-(3,5-dicarboxybenzyloxy)-3-pyridine carboxylic acid; DMA = dimethyl acetamide; DMF = dimethyl formamide) have been synthesized under hydro(solvo)thermal conditions and characterized by X-ray crystallography. The five compounds represent extended coordination-polymeric assemblies; double interweaving of the metal–organic frameworks has been observed in 3–5. A diverse metal–ligand coordination schemes have been observed, mono- (in 2–5), di- (in 2) and tri-nuclear (in 1). Thermal analysis (TGA and DTA) features of 1–5 and solid-state variable temperature magnetic susceptibility data of 3, 4 and 5 are reported as well. The magnetic susceptibility data reveal weak anti-ferromagnetic coupling between the metal centers in these complexes.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...