Polymorphism in metformin embonate salt – recurrence of dimeric and tetrameric guanidinium–carboxylate synthons†
Abstract
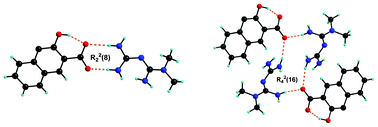
In this manuscript, we discuss two novel polymorphic crystal structures of metformin–embonate, a drug widely known for its use in anti-diabetic treatments. Form I crystallizes in the monoclinic P21/c space group and form II in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. Both polymorphs contain 2 : 1 stoichiometry of metformin and embonic acid. Proton transfer results in a guanidinium carboxylate ion pair which has well aligned donor and acceptor groups for effective hydrogen bonding. Two types of guanidinium–carboxylate variations are observed in the polymorphs. The first is the dimer motif, R22 (8), and the second is the tetramer motif, R24 (16). In addition, there is a self complementary N–H⋯N dimer motif, R22 (8), between the two guanidinium groups. It is interesting to note that both polymorphs contain these three synthons but the arrangement of the synthons differs between them. Predominantly, these are packing polymorphs. The highly recurring nature or robustness of the synthons is likely to arise from the structural complementary nature of the interacting groups and due to the charge assistance in the ion pair. The subtle hydrogen bonding differences in the polymorphs are corroborated with infrared and Raman spectroscopic observations. The polymorph stability relationships are established through DSC, a slurry method, equilibrium solubility and IDR measurements. The results indicate that the polymorphs have an enantiotropic relationship – form I is the high temperature stable form and form II is the room temperature stable form. Form II, the more stable form at RT, has lower solubility and lower dissolution rates compared to form I. The guanidine–carboxylate dimer synthon is estimated to be highly robust in the CSD synthon analysis (72.3% in the presence of competing groups and 96.3% in their absence), which can be reliably explored for crystal engineering applications.
space group. Both polymorphs contain 2 : 1 stoichiometry of metformin and embonic acid. Proton transfer results in a guanidinium carboxylate ion pair which has well aligned donor and acceptor groups for effective hydrogen bonding. Two types of guanidinium–carboxylate variations are observed in the polymorphs. The first is the dimer motif, R22 (8), and the second is the tetramer motif, R24 (16). In addition, there is a self complementary N–H⋯N dimer motif, R22 (8), between the two guanidinium groups. It is interesting to note that both polymorphs contain these three synthons but the arrangement of the synthons differs between them. Predominantly, these are packing polymorphs. The highly recurring nature or robustness of the synthons is likely to arise from the structural complementary nature of the interacting groups and due to the charge assistance in the ion pair. The subtle hydrogen bonding differences in the polymorphs are corroborated with infrared and Raman spectroscopic observations. The polymorph stability relationships are established through DSC, a slurry method, equilibrium solubility and IDR measurements. The results indicate that the polymorphs have an enantiotropic relationship – form I is the high temperature stable form and form II is the room temperature stable form. Form II, the more stable form at RT, has lower solubility and lower dissolution rates compared to form I. The guanidine–carboxylate dimer synthon is estimated to be highly robust in the CSD synthon analysis (72.3% in the presence of competing groups and 96.3% in their absence), which can be reliably explored for crystal engineering applications.

 Please wait while we load your content...
Please wait while we load your content...