Calcite formation by hydrothermal carbonation of portlandite: complementary insights from experiment and simulation
Abstract
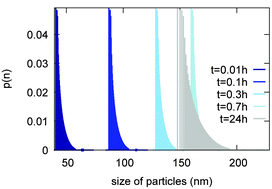
The present study complements experimental results of calcite nanoparticle formation by hydrothermal carbonation of calcium hydroxide by a simulation strategy, in which both the chemical evolution of the aqueous solution and the solid phases – dissolution of portlandite and nucleation and growth of secondary calcite particles – are considered. The simulation is performed with the help of the NANOKIN code. It includes a full treatment of speciation processes in the aqueous solution, a rate equation for the dissolution of primary minerals, and a full account of nucleation and growth processes during the formation of new particles. This strategy has allowed us to decipher the various steps in the mineral transformation and solution evolution. The comparison between experiment and simulation puts strong constraints on simulation parameters, while modeling can give information on in situ conditions, currently not often available experimentally.

 Please wait while we load your content...
Please wait while we load your content...