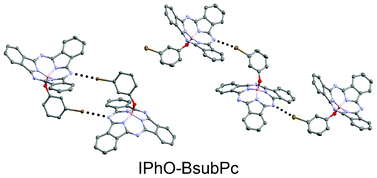
We report the synthesis and crystal structure analysis of a series of halo-substituted phenoxy–boron subphthalocyanine (phenoxy–BsubPc) derivatives, including meta-halogenated-phenoxy–BsubPcs, ortho-halogenated-phenoxy–BsubPcs, pentabromophenoxy–BsubPc and pentachlorophenoxy–BsubPc. We have found that the majority of the resulting solid state arrangements contain definite halogen bonds, which have not previously been seen in the solid state arrangement of phenoxy–BsubPcs. We observed that the presence and strength of halogen bonds are dependent upon the particular halogen atom. We used semi-empirical methods to calculate the approximate halogen bond strength for the meta-halogenated-phenoxy–BsubPcs. This comparative approach is useful in cases where a structurally-similar isomer exists with a solid state arrangement that is similar, but which differs by exactly one type of significant intermolecular interaction. We also observed that the tendency to form halogen bonds in phenoxy–BsubPcs depends on the position of the halogen atom, and increases in the order: para < ortho < meta. The presence of halogen bonds facilitates the arrangement of BsubPc into networks of closely associated molecules extending into two and three dimensions, resulting in solid state arrangements which are noteworthy exceptions to the typical phenoxy–BsubPc packing motifs.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...